When performing ELISA assays, have you encountered issues such as poor standard-curve linearity, sample OD values exceeding the detection range, or poor reproducibility? In many cases, the root cause is skipping a critical step: the pilot (preliminary) experiment. Below is a practical guide on how to conduct an effective pilot experiment to ensure reliable results and avoid unnecessary setbacks in subsequent assays.

Why Is a Pilot Experiment Necessary?
A pilot experiment is not an “extra burden”; it is the compass of ELISA testing. Its value lies in three key aspects:
1. Estimating the target analyte concentration range
A pilot experiment helps determine the approximate concentration of the target analyte in the samples, preventing inaccurate results caused by values that are too high or too low in the formal experiment.
For example, when using a human IL-6 ELISA kit to measure IL-6 levels in cell culture supernatants (as shown in the figure), the standard curve exhibited excellent linearity (R² > 0.99). However, all sample OD values exceeded the OD corresponding to the highest standard concentration (2.386), making it impossible to calculate sample concentrations. This issue could have been avoided with a pilot experiment.
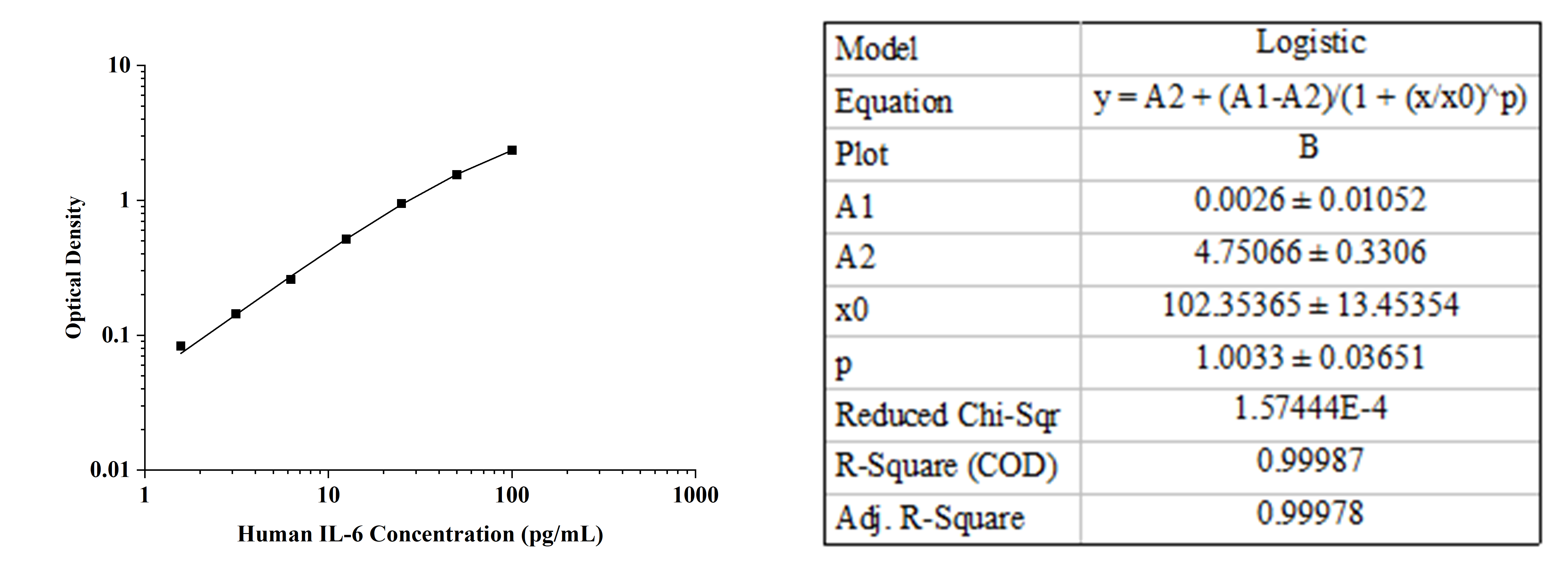
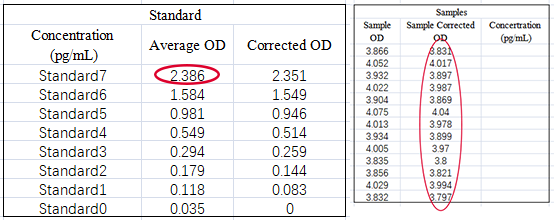

2. Verifying kit–sample compatibility and identifying interference factors
Some samples may contain interfering factors such as hemolysis, impurities, or cross-reactive substances. These issues can be identified and addressed during the pilot experiment, thereby reducing waste of samples, reagents, and time.
If the pilot experiment is skipped and abnormal results arise in the formal assay (e.g., poor standard-curve linearity or large OD fluctuations), it becomes difficult to determine whether the problem lies with the target concentration, kit performance, or the sample itself—potentially stalling the entire experiment.
3. Improving feasibility, efficiency, and accuracy
By testing a small number of representative samples on a limited scale, the pilot experiment increases the likelihood that the formal experiment will succeed on the first attempt.
How Should a Pilot Experiment Be Conducted?
Reed Biotech ELISA kits are supplied with pre-coated, detachable strips, allowing users to take only the required number of wells while storing the remaining strips in aluminum foil at −20 °C. This design provides an excellent foundation for pilot experiments.
In practice, researchers often consult the literature to estimate the expected concentration range of the target analyte. However, for complex sample types—such as cell culture supernatants, tissue homogenates, cerebrospinal fluid, or bronchoalveolar lavage fluid—differences in sample source, culture conditions, collection, and storage can lead to substantial deviations from published values. In such cases, literature data may not be reliable, making the pilot experiment indispensable.
The core objective of an ELISA pilot experiment is to determine the optimal sample dilution factor and confirm compatibility with the assay system. The design should include both the standard curve and test samples to ensure accuracy and reliability in the subsequent formal experiment.
To efficiently identify the optimal dilution, it is recommended to use wide dilution gradients, such as 10×, 100×, and 1000×. If dilution steps are too narrow (e.g., 2× or 5×), differences in OD values between dilutions may lack statistical significance, making it difficult to define the appropriate dilution range and potentially delaying progress.
If the study includes multiple experimental groups (e.g., blank control, model, treatment groups), representative samples from each group should be included in the pilot experiment. Target protein levels can vary substantially between groups, and results from a single group may not be applicable to the entire study.
For example, in a mouse inflammation model, interleukin-6 (IL-6) is a key inflammatory cytokine. Its concentration is typically lowest in the blank control group and markedly elevated in the model group. Including samples from these extreme groups in the pilot experiment allows rapid identification of a dilution range suitable for all groups, thereby improving efficiency and providing a solid scientific basis for optimizing the formal experimental protocol.
How Should Pilot Experiment Results Be Analyzed?
Based on the relationship between standard concentrations and OD values, generate a standard curve and identify the sample dilution at which OD values fall within the linear range of the curve. This dilution is then used in the formal experiment.
For example, in the pilot experiment shown, a serum sample was tested at 50× and 1000× dilutions alongside the standards. The undiluted sample produced an OD value above the maximum standard. At 1000× dilution, the OD value approached background levels, while at 50× dilution, the sample OD fell within the standard curve range. Therefore, a 50× dilution was determined to be the appropriate dilution for the formal assay.
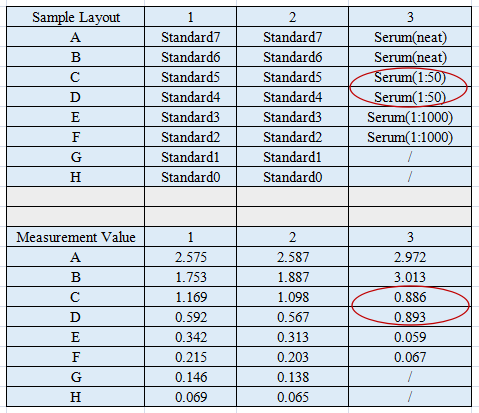
Although simple, a pilot experiment can directly determine the success or failure of an ELISA assay. By following the approaches outlined above, you can complete pilot experiments efficiently and achieve reliable results in your formal experiments on the first attempt. If you encounter any issues during pilot testing or ELISA analysis, please feel free to contact us—our technical support team is always available to assist you.
