Title:SENP6 Restrains NLRP3 Inflammasome Activation via DeSUMOylation-Driven K48-Linked Ubiquitination of NLRP3 in Acute Lung Injury
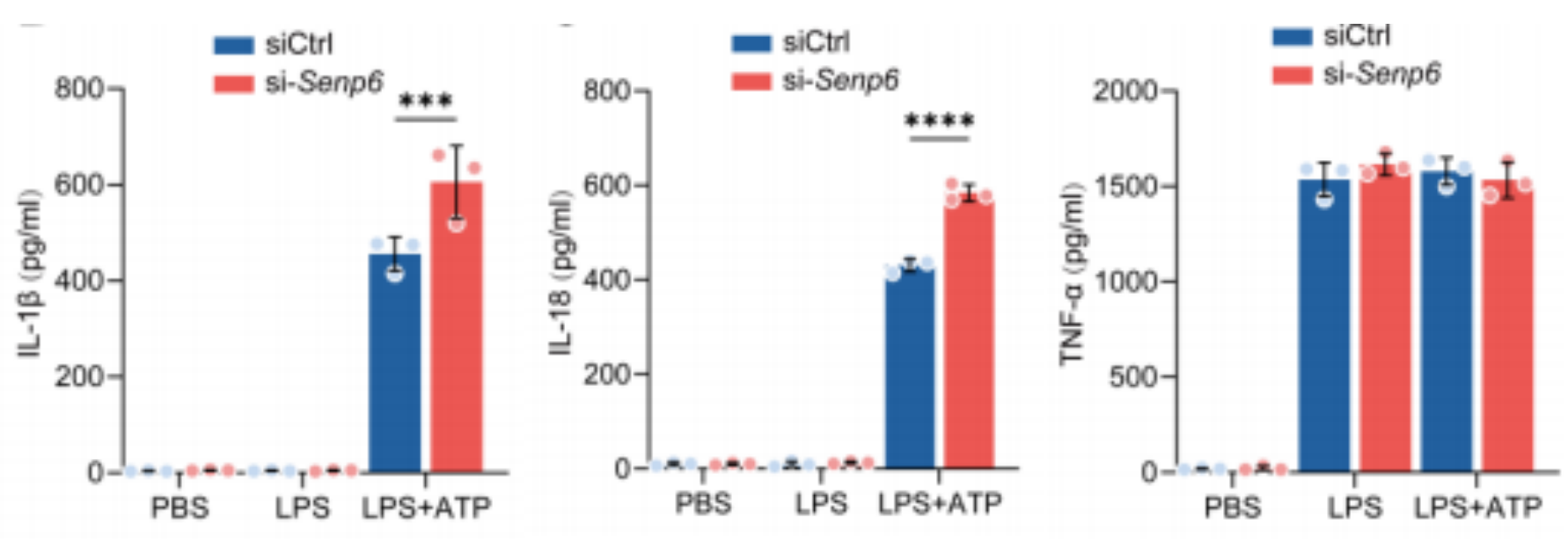
Levels of IL-1β, IL-18, and TNF-α in the supernatants of peritoneal macrophages from SENP6^fl/fl and SENP6^fl/fl Lyz2-Cre mice following LPS priming and ATP activation.
Affiliation:The First Affiliated Hospital Of Shandong First Medcial Unversity
DOI: 10.34133/research.106

Related Products:
Mouse IL-1βELISA Kit (RE1074M)
Mouse IL-18 ELISA Kit (RE1123M)
Mouse TNF-α ELISA Kit (RE1060M)
Sample Types:
Cell culture supernatant
Serum
Bronchoalveolar lavage fluid
Abstract:
The NLRP3 inflammasome is a pivotal component of the innate immune system that responds to infection and cellular damage. Dysregulation of NLRP3 activation is implicated in a wide range of inflammatory diseases; however, the mechanisms governing its activation remain incompletely understood. Recent studies have highlighted the critical roles of post-translational modifications, such as ubiquitination and SUMOylation, in regulating inflammasome activity.
In this study, we demonstrate that SENP6, a SUMO-specific protease, negatively regulates NLRP3 inflammasome activation by promoting K48-linked polyubiquitination of NLRP3. SENP6-deficient macrophages exhibit enhanced NLRP3 activation and increased secretion of IL-1β and IL-18, leading to amplified inflammatory responses. Mechanistically, SENP6 interacts directly with NLRP3 and facilitates its degradation through K48-linked polyubiquitination via the autophagy–lysosome pathway.
We further identify that SENP6 deSUMOylates NLRP3 at specific lysine residues (K23, K204, and K689), a process that is essential for maintaining NLRP3 protein homeostasis. In addition, SENP6 recruits the E3 ubiquitin ligase MARCHF7 to promote NLRP3 ubiquitination and subsequent degradation.
In vivo, SENP6 deficiency exacerbates NLRP3 activation and pulmonary inflammation in lipopolysaccharide (LPS)-induced endotoxemia-associated acute lung injury, and intensifies inflammatory responses in alum-induced peritonitis.
Collectively, these findings uncover a previously unrecognized mechanism by which SENP6 regulates NLRP3 inflammasome activation through coordinated control of SUMOylation, ubiquitination, and protein degradation, and provide new insights into potential therapeutic strategies for inflammasome-related inflammatory pathologies.
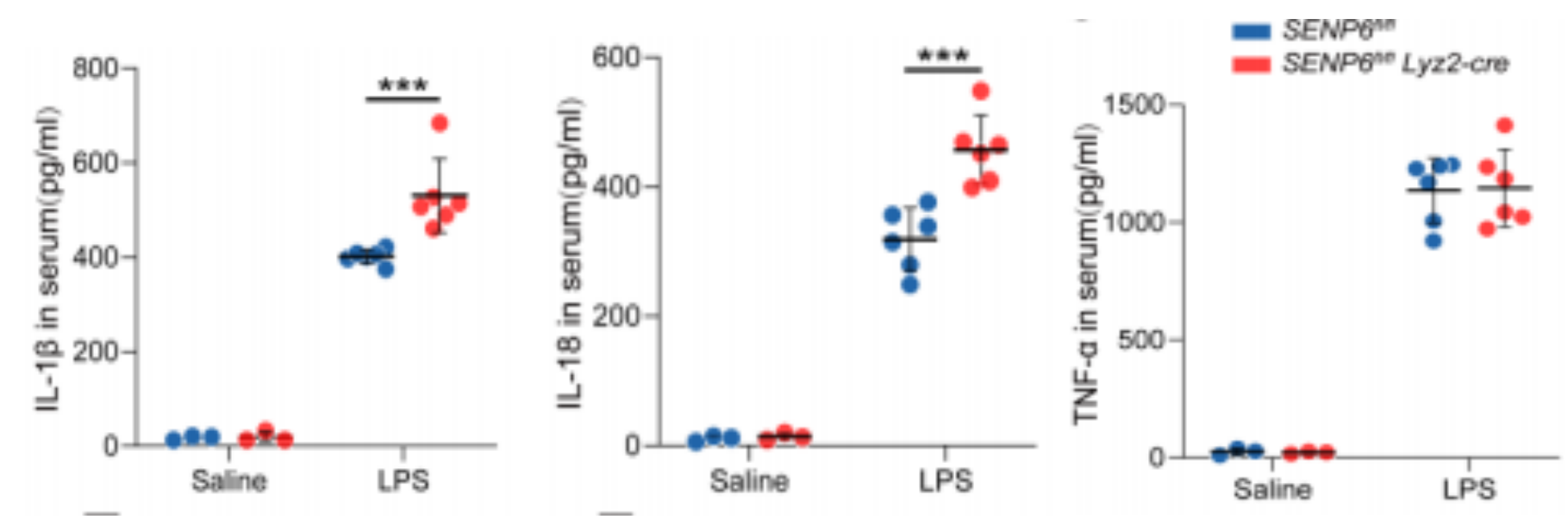
Serum levels of IL-1β, IL-18, and TNF-α were measured 12 hours after intraperitoneal injection of either saline (100 μL) or LPS (25 mg/kg) in SENP6^fl/fl and SENP6^fl/fl Lyz2-Cre mice.
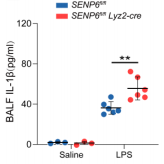
Levels of IL-1β in bronchoalveolar lavage fluid from SENP6^fl/fl and SENP6^fl/fl Lyz2-Cre mice 12 hours after intraperitoneal LPS injection.
