When memories are quietly "stolen," how can I "protect you"?
Alzheimer's disease was first discovered in 1906 by German physician Dr. Alois Alzheimer. He described the brain changes of an elderly European woman with memory loss and mental confusion, reporting the main features of "amyloid plaques" and "neurofibrillar tangles" in her brain[1]. To commemorate Dr. Alzheimer's contribution to the disease, it was named Alzheimer. More than a century later, there are more than 55 million patients worldwide, and about 10 million of them are in China.
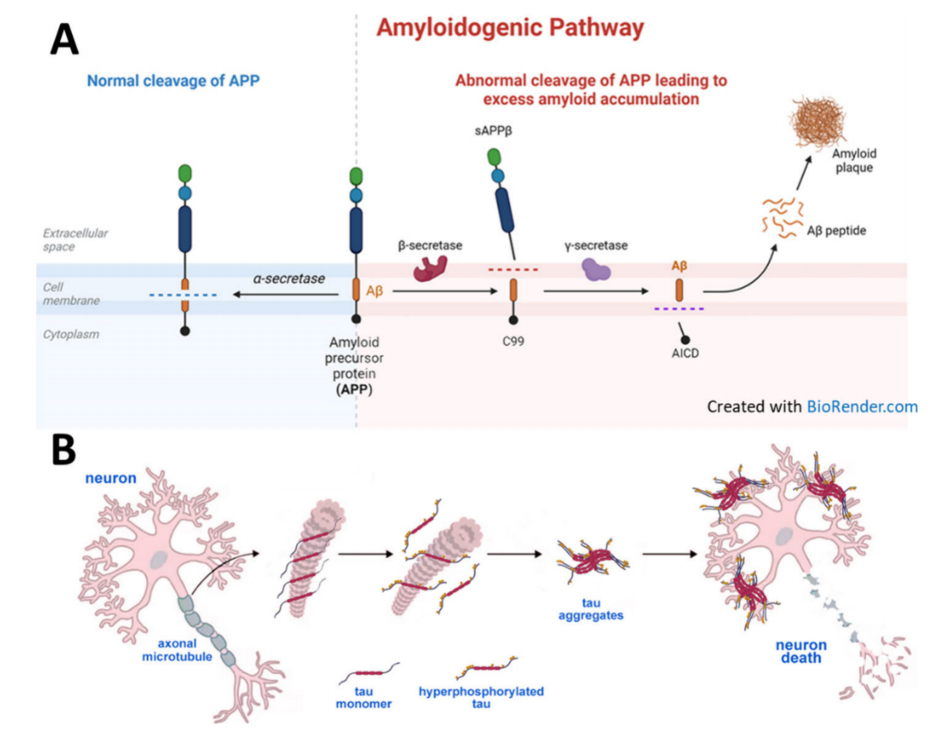
A.Normal and abnormal cleavage of amyloid precursor protein (APP);
B.tau protein forms neurofibrillary tangles, leading to neuronal death [2]
I. More Than Just Forgetting——The Trilogy of Memory Slippage
The development of Alzheimer's disease is like a slow downhill slide, often divided into three stages: preclinical stage - mild cognitive impairment (MCI) - dementia [1]. Patients in the preclinical stage have no symptoms, but cerebrospinal fluid examination may reveal: decreased Aβ 42, increased total tau and p-tau181, or positive amyloid PET imaging. These changes can occur 10-20 years before clinical diagnosis [3]. In the MCI stage, the most obvious manifestations are: recent memory decline – asking "what did you eat for lunch" even though you have just eaten; decreased speed and accuracy in performing complex tasks (such as financial management), but still able to take care of daily life [1,3]. After developing into dementia, language, executive function, and motor function all decline. Naming aphasia, spatial perception deteriorates, personality and mood also change, depression and anxiety occur, and some patients experience delusions or hallucinations [1]. In the moderate stage, patients frequently ask repeated questions and require assistance with dressing and bathing; in the severe stage, they become unsteady on their feet, have difficulty swallowing, and experience urinary and fecal incontinence, eventually passing away due to infection and multiple organ failure [1]. Throughout the course of the disease, brain imaging also shows synchronous atrophy: MRI shows that the hippocampus shrinks by more than 5% per year, and PET-CT shows that amyloid plaques are distributed in a "scattering of flowers" pattern, and tau tangles spread like "flames" from the medial temporal lobe to the parietal-occipital region [3,4].
II. Treatment——A Three-Stage Leap from “Symptomatic” to “Etiological”
The first batch of drugs used clinically—cholinesterase inhibitors (donepezil, rivastigmine, galantamine) and NMDA receptor antagonists (memantine)—temporarily increased the concentration of acetylcholine in the brain or regulated glutamatergic transmission, resulting in mild improvement of cognitive scores within 6-12 months, but could not block the underlying pathological process [4]. With the clinical validation of the “amyloid cascade hypothesis,” three anti-Aβ monoclonal antibody drugs were launched in succession from 2021 to 2025. Aducanumab can clear plaques, but its clinical application is limited due to controversy over its efficacy; Lecanemab slowed the decline rate of CDR-SB score by 27% in trials and reduced the amyloid burden in the brain to below the negative threshold, but was accompanied by a 12.6% incidence of cerebral edema [4,5]; Donanemab is mainly for mild to moderate tau patients, slowing the decline of iADRS score by 35%, but the ARIA-E rate was as high as 24% [4]. The common mechanism of these drugs is that the antibody Fc fragment binds to the FcγR of microglia in the brain, mediating phagocytosis and clearance of Aβ plaques to reduce synaptic toxicity and inflammatory response [5,6]. However, single-target strategies are clearly insufficient, and scientists are currently testing synergistic approaches.
III. Challenges and Opportunities——Finding a Bright Future Amidst Difficulties
The road to treating Alzheimer's disease is not easy, but the more severe the challenges, the clearer the opportunities.
1. Ceiling of Efficacy -- Breaking Through with “Multi-Target Combination”
Existing anti-Aβ monoclonal antibodies can only slow down cognitive decline by an average of 25–30%, with the remaining 70% of decline related to tau tangles, cerebrovascular lesions, chronic inflammation, and persistent synaptic loss. The solution is synergistic therapy: anti-Aβ antibody + anti-tau oligonucleotide + NLRP3 inflammasome inhibitor + GLP-1 receptor agonist that improves brain energy metabolism. Multiple phase II/III trials have been initiated globally [4].
2.Side effects of ARIA--“Brain shuttle” reduces risk
When antibodies bind to Aβ deposited in the blood vessel wall, they activate complement and microglia, causing vasogenic edema or microbleeds, increasing the risk of ARIA-E by 3-4 times, requiring multiple MRI monitoring in clinical practice [4,5]. However, the “brain shuttle” technology modifies the Fc segment of the antibody to bind to the transferrin receptor in the cerebral vascular endothelium, achieving active transport, increasing the drug concentration in the brain by 5-8 times, and reducing ARIA-like pathology by >70% in mouse models [6].
3.Delayed diagnosis -“Early plasma screening” enters the community.
When obvious symptoms appear, about 30% of the neurons in the medial temporal lobe have died, and the gold standard for diagnosis - amyloid PET or cerebrospinal fluid testing - is expensive or invasive [3]. With the advancement of high-sensitivity analysis platforms and detection technologies, the diagnostic accuracy and reliability of blood biomarkers have been continuously improved. Ultrasensitive single-molecule array and mass spectrometry can “predict” brain pathology 10-20 years in advance with plasma p-tau217+Aβ 42/40 combination, and the cost has been reduced to less than 100 yuan. It is expected to be included in the medical insurance screening catalog in 2026, making “memory checkup” as simple as measuring blood pressure [1,3].
4.Population differences—— “Multi-omics map” for precise subtyping
Plasma p-tau217, Aβ 42/40 and other cut values are mainly based on European and American white cohorts, and there is insufficient data for East Asian and African populations [4]. Integrating GWAS-proteomics-metabolomics can construct an individualized “AD multi-omics risk score” to achieve “one person, one map” for precise intervention [1].
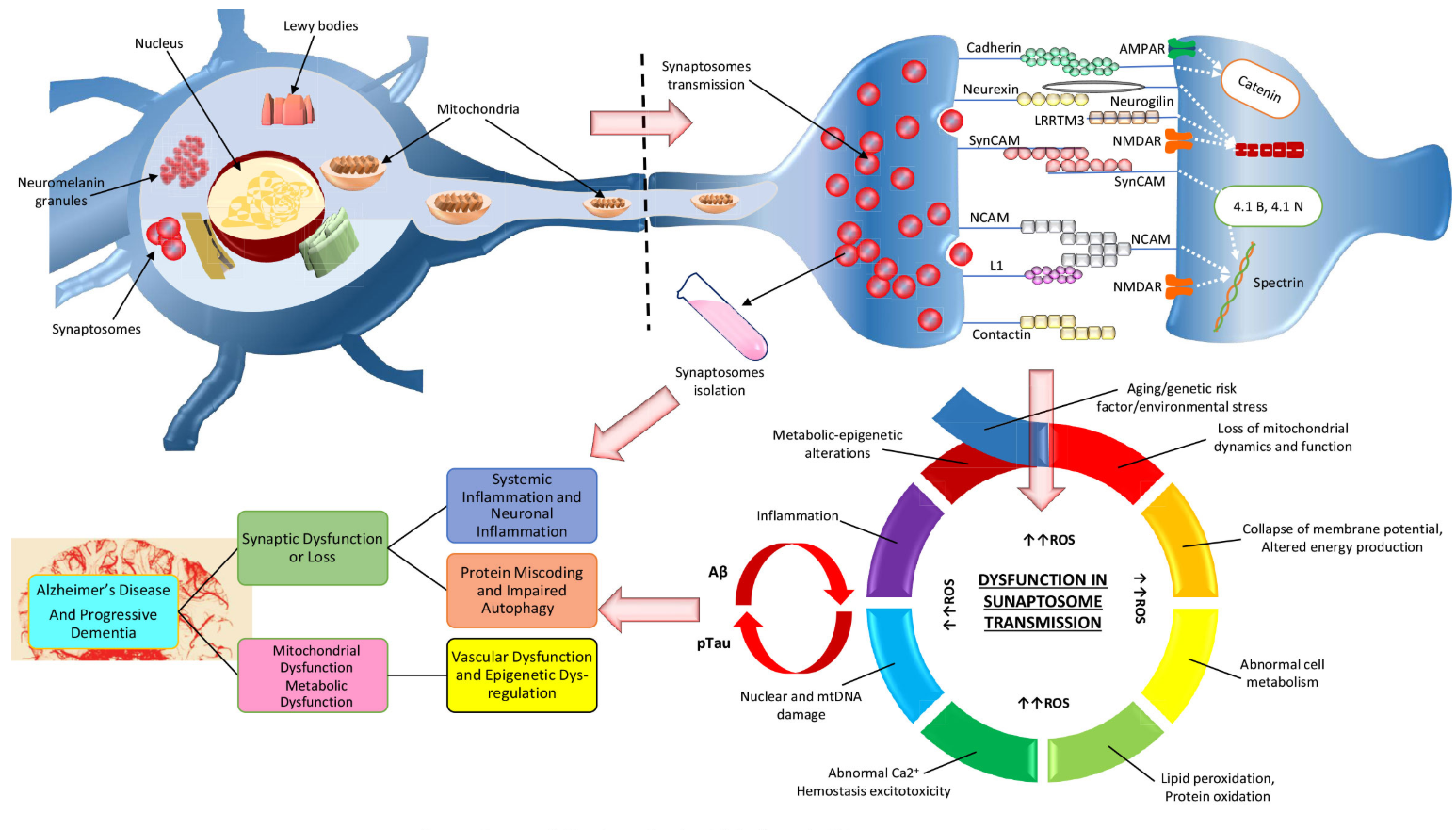
Synaptosomal proteomics in Alzheimer's disease [7].
5. Lifestyle – “Four Things to Do Every Day” to Reduce Risk
Adhering to the MIND diet (leafy greens, nuts, olive oil, fish), 150 minutes of moderate-intensity aerobic + resistance training per week, and social and cognitive training can reduce the risk of APOE ε4 carriers by 40% [1].
6.“Medical Insurance + Network” – Connecting the Last Mile
The National Health Commission has included early AD screening in the “Healthy China 2030” special action; Shanghai, Zhejiang, Beijing and other places are piloting a three-level network of “memory clinic-community-family”, which is planned to cover 80% of people over 60 years old by 2027 [4].
Conclusion:
With the combined efforts of early screening, brain shuttle therapy, combined treatment, lifestyle modifications, and policies, we believe that in the future, Alzheimer's disease will no longer be a "long farewell," but rather a "continuous protection."
References
1. Krishnamurthy HK, Jayaraman V, Krishna K, et al. An overview of the genes and biomarkers in Alzheimer's disease. Ageing Res Rev. 2025;104:102599. doi:10.1016/j.arr.2024.102599
2.Tenchov R, Sasso JM, Zhou QA. Alzheimer's Disease: Exploring the Landscape of Cognitive Decline. ACS Chem Neurosci. 2024;15(21):3800-3827. doi:10.1021/acschemneuro.4c00339
3. Humpel C. Identifying and validating biomarkers for Alzheimer's disease. Trends Biotechnol. 2011;29(1):26-32. doi:10.1016/j.tibtech.2010.09.007
4. Hardy J. Alzheimer's Disease: Treatment Challenges for the Future. J Neurochem. 2025;169(8):e70176. doi:10.1111/jnc.70176
5. Söderberg L, et al. Lecanemab, Aducanumab, and Gantenerumab - Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer's Disease. Neurotherapeutics. 2023;20(1):195-206. doi:10.1007/s13311-022-01308-6
6. Xing M, Song W. Improving Alzheimer's disease immunotherapy. Science. 2025;389(6760):571-572. doi:10.1126/science.adz8959
7. Ahmad F, Haque S, Chavda V, Ashraf GM. Recent Advances in Synaptosomal Proteomics in Alzheimer's Disease. Curr Protein Pept Sci. 2021;22(6):479-492. doi:10.2174/1389203722666210618110233
