When patients with hyperlipidemia suffer from infectious bone defects (IBD), are traditional treatments helpless? A team from Shandong First Medical University has published a groundbreaking study in the Chemical Engineering Journal: they have developed an injectable multifunctional hydrogel that integrates four key effects—antibacterial, antioxidant, immunomodulatory, and adipogenesis-inhibiting—bringing new hope for refractory bone defects.
1.Clinical Pain Point: When Infection Meets Hyperlipidemia, Bone Defect Repair Becomes a "Daunting Challenge"
Infectious bone defect (IBD) is already a "tough nut to crack" in orthopedics and oral and maxillofacial surgery—bacterial biofilms are stubbornly drug-resistant, local inflammation persists, and bone regeneration is severely delayed. What's more tricky is that an increasing number of patients are complicated with hyperlipidemia: their immunity is impaired, stem cells "differentiate abnormally", reactive oxygen species (ROS) are overproduced, and bone marrow mesenchymal stem cells (BMSCs) do not differentiate towards osteogenesis but instead transform into adipocytes in large quantities. Under the triple blow of inflammation-oxidative stress-lipid metabolism disorder, the traditional "debridement + antibiotics" scheme is greatly compromised.
2.Innovative Strategy: The "Four-Fold Cultivation" of a Hydrogel
Faced with this complex microenvironment, the research team proposed a new idea of "synergistically remodeling the microenvironment" and designed an intelligent nanocomposite hydrogel—SMAL:
• Chitosan-aldehyde starch hydrogel matrix: Injectable, self-healing, and pH-responsive degradable.
• Hollow mesoporous MnO₂ nanospheres: Act as "ROS scavengers" to efficiently remove hydrogen peroxide and superoxide anions.
• Silver ions + Lovastatin: Silver ions sterilize, while statin drugs simultaneously lower lipids, reduce inflammation, and promote osteogenesis.
The hydrogel degrades in response to the slightly acidic microenvironment (pH~5.5) at the infection site, enabling targeted and intelligent drug release and avoiding systemic side effects.
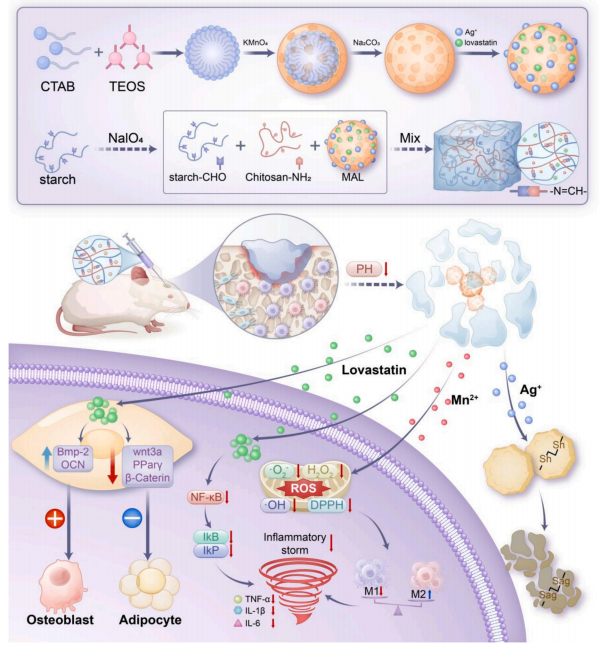
Schematic diagram of the SMAL hydrogel system regulating the bone metabolic microenvironment in infectious bone defects.
3.Solid Data: The "Highlight Moments" in the Laboratory
Laboratory verification showed that the bacteriostatic rate against methicillin-resistant Staphylococcus aureus (MRSA) exceeded 99%, and scanning electron microscopy revealed bacterial cell membrane rupture and content leakage. More impressively, it can effectively destroy bacterial biofilms—the culprit behind 90% of chronic infections. In a high oxidative stress environment simulated by H₂O₂, the intracellular ROS level in the SMAL group decreased by nearly 70%, and mitochondrial function returned to normal. DPPH free radical scavenging experiments showed that its antioxidant capacity increased in a dose-dependent manner. Meanwhile, macrophages "turned over a new leaf": ELISA tests showed that pro-inflammatory factors TNF-α, IL-1β, and IL-6 decreased significantly, anti-inflammatory factor IL-10 increased significantly, and macrophages polarized from M1 to M2 type. In a high-lipid environment, the ALP activity in the SMAL group increased by 3 times, with dense mineralized nodules (Alizarin Red staining), while the control group showed numerous lipid droplets in Oil Red O staining.
The research team further established a triple model of hyperlipidemia + MRSA infection + skull defect. Micro-CT results after 8 weeks of SMAL hydrogel implantation were striking: the bone volume fraction (BV/TV) was 3.2 times that of the control group, bone mineral density (BMD) increased by nearly 2 times, the defect area was almost completely filled with new bone, and seamlessly connected with the surrounding normal bone. Histological staining showed ordered arrangement of collagen fibers, high expression of the osteogenic marker BMP-2, and almost disappearance of the adipogenic marker PPARγ. More surprisingly, the blood lipid levels and systemic inflammatory indicators of the rats also decreased, achieving "local treatment with systemic benefits".
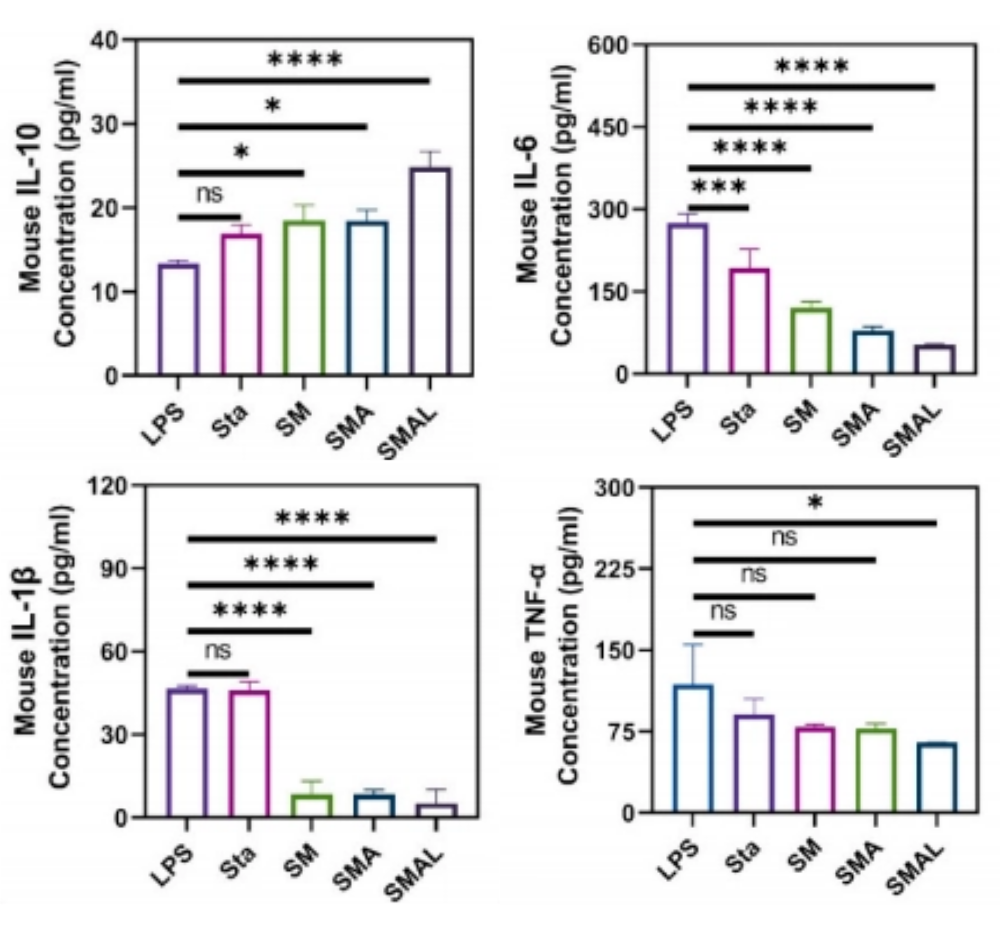
ELISA assays were performed to measure the levels of TNF-α (M1), IL-1β(M1), IL-6(M1), IL-10 (M2) in the supernatants from different treatment groups
4.Clinical Significance: More Than Just Bone Defects
This study is the first to integrate lipid-lowering drugs into bone repair materials, pioneering an IBD therapy for patients with metabolic abnormalities, and realizing a local-systemic combined treatment strategy. In the future, it may benefit the treatment of mandibular osteomyelitis, diabetic foot complicated with infection, nonunion of fractures in obese patients and other difficult problems.
5.Research Inspiration: Good Tools Facilitate Good Research
In this study, all ELISA kits used were from Reed Biotech. The accuracy of these data directly supports the core highlight of "immunomodulation". Advantages of Reed Biotech's ELISA kits:
✅ High specificity: Low cross-reactivity, suitable for complex biological samples.
✅ Wide linear range: Covering concentrations from pg/mL to ng/mL.
✅ Inter-batch stability: Reproducible data in multi-center studies.
If you are conducting research related to inflammation, immunity, or metabolism, try this ELISA kit validated by top journal studies to make your data more convincing!
|
Name |
Catalog Number |
Sensitivity |
Detection Range |
|
Mouse TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit |
RE1060M |
0.27 pg/mL |
0.94-60 pg/mL |
|
Mouse IL-1β(Interleukin 1 Beta) ELISA Kit |
RE1074M |
2.35 pg/mL |
3.91-250 pg/mL |
|
Mouse IL-6(Interleukin 6) ELISA Kit |
RE3186M |
0.2 pg/mL |
1.56-100 pg/mL |
|
Mouse IL-10(Interleukin 10) ELISA Kit |
RE3187M |
9.38 pg/mL |
15.63-1000 pg/mL |
