If we compare a cell to a bustling city, solute carrier (SLC) transport proteins are like the "logistics hubs" scattered throughout the city. They determine which nutrients can enter the city, which metabolic wastes must be transported away, and even directly participate in the city's "security" and "planning." A recent review shows that the human genome encodes more than 400 SLC members, widely distributed in five major "transportation hubs"—the kidneys, brain, liver, intestines, and heart—and are closely related to various chronic diseases such as diabetes, hypertension, gout, and Alzheimer's disease.
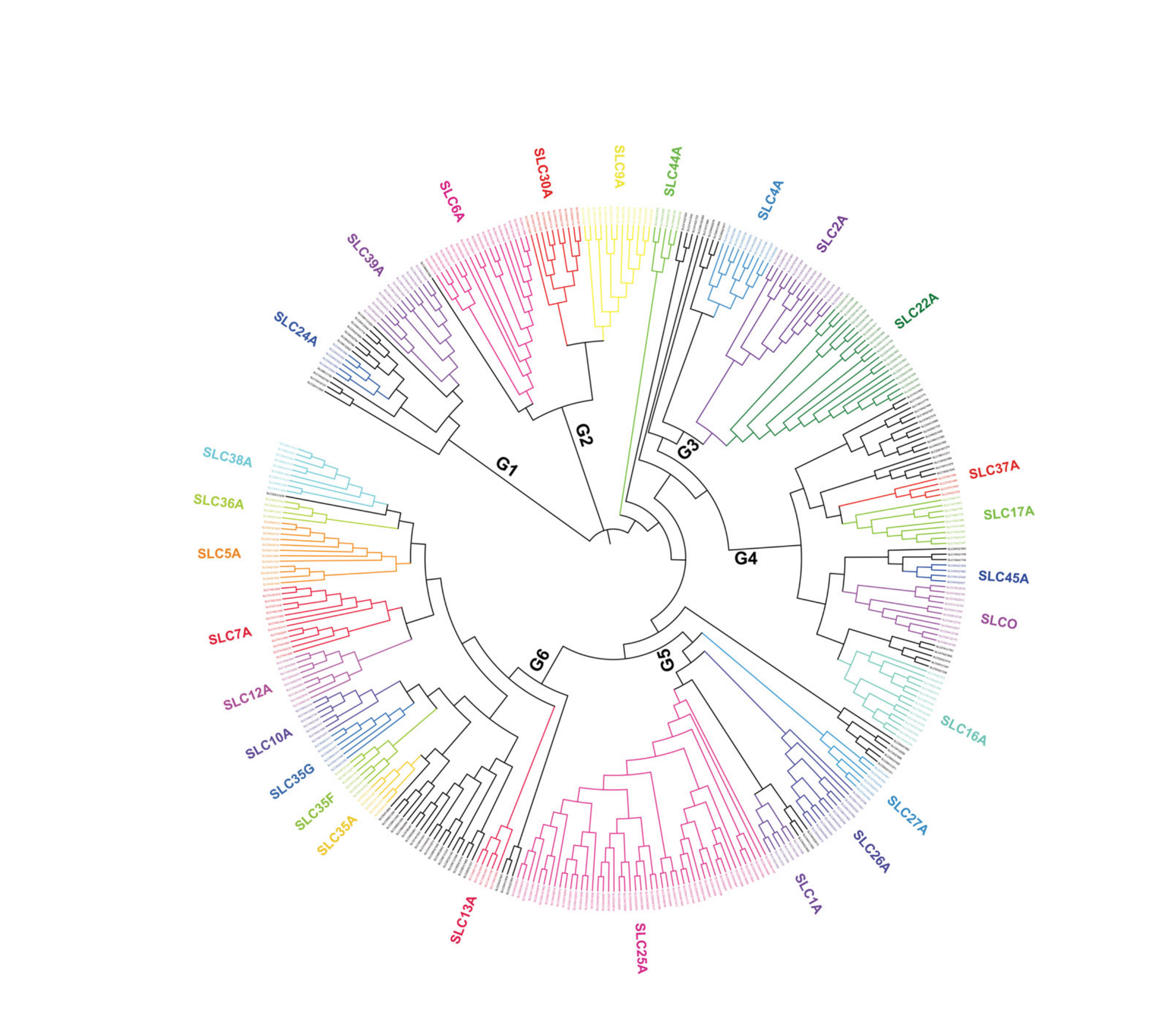
Figure 1 | SLC superfamily phylogenetic tree (Zhang et al., 2019)
01. Four modes of transportation, one "city pass"
SLC proteins facilitate transmembrane transport through four mechanisms:
- lCotransporter: For example, SGLT1/2 (SLC5A1/2) uses the sodium ion gradient to "drag" glucose into cells, a core mechanism for renal glucose reabsorption.
- lExchanger: For example, NHE3 (SLC9A3) couples Na⁺ influx with H⁺ efflux in the gut, maintaining acid-base balance.
- lFacilitated diffusion: For example, GLUT2 (SLC2A2) allows glucose to rapidly enter and exit hepatocytes down its concentration gradient.
- lOrphan transport: For example, SLC38A9 transports leucine from lysosomes to the cytoplasm, directly activating the mTORC1 nutrient sensing pathway.
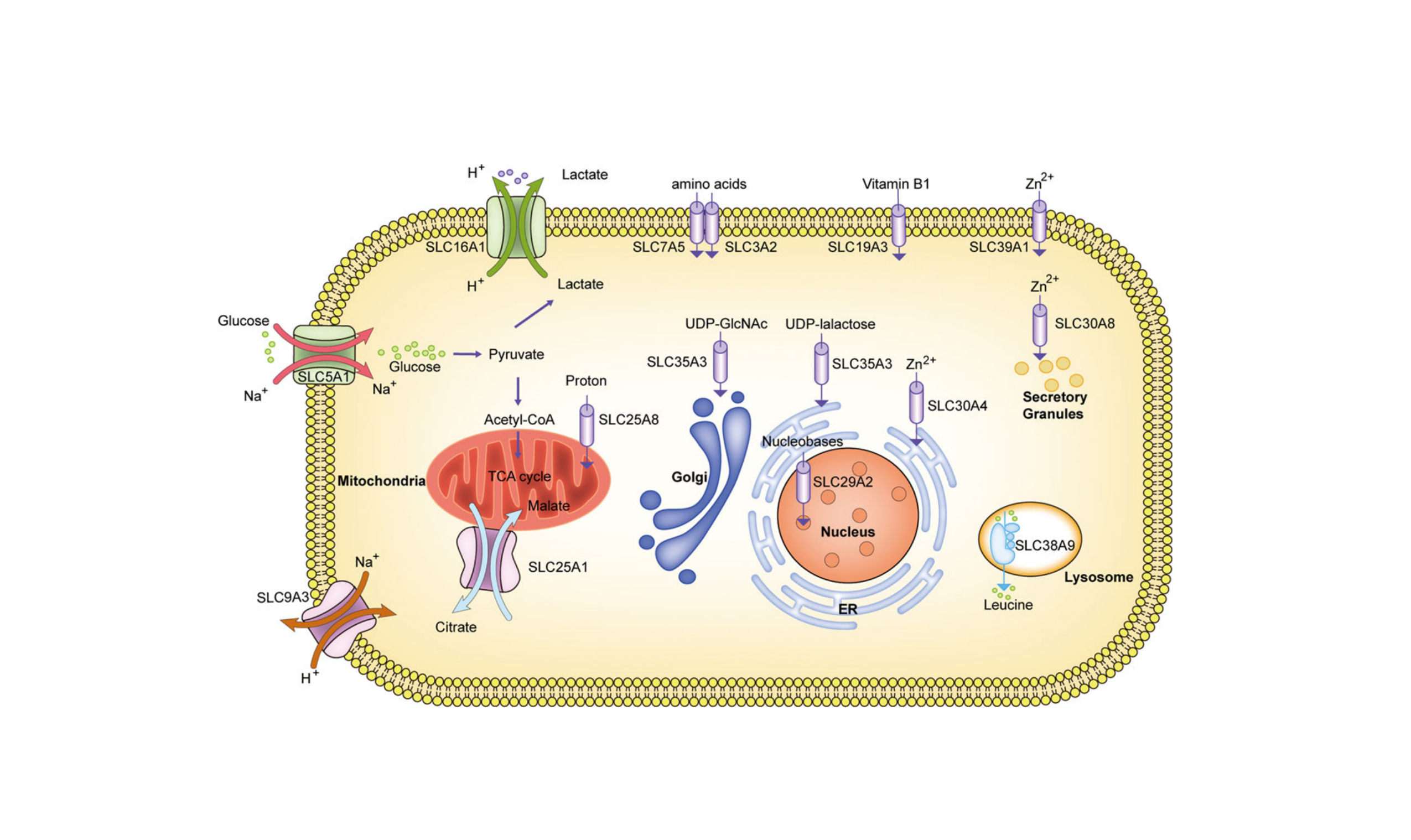
Figure 2 | Four modes of transport for SLC (Zhang et al., 2019)
02. Five major organs, a“disease map”
Kidney: Uric acid transporters such as SLC2A9 and SLC22A12 determine gout risk; SLC12A3 mutations can cause Gitelman syndrome-type hypokalemia.
Brain: SLC6A4 (5-HTT) affects mood; SLC24A4 is associated with Alzheimer's disease and decreased sense of smell.
Liver: SLC30A8 (ZnT8) regulates zinc ions in insulin granules; mutations increase the risk of type 2 diabetes by 30%.
Intestine: SLC15A1 (PepT1) is the "first-line" for oral medications, determining the absorption of oligopeptides and β-lactam antibiotics.
Heart: SLC4A7 (NBCn1) maintains myocardial pH; mice lacking this feature exhibit mild hypertension and arterial remodeling.
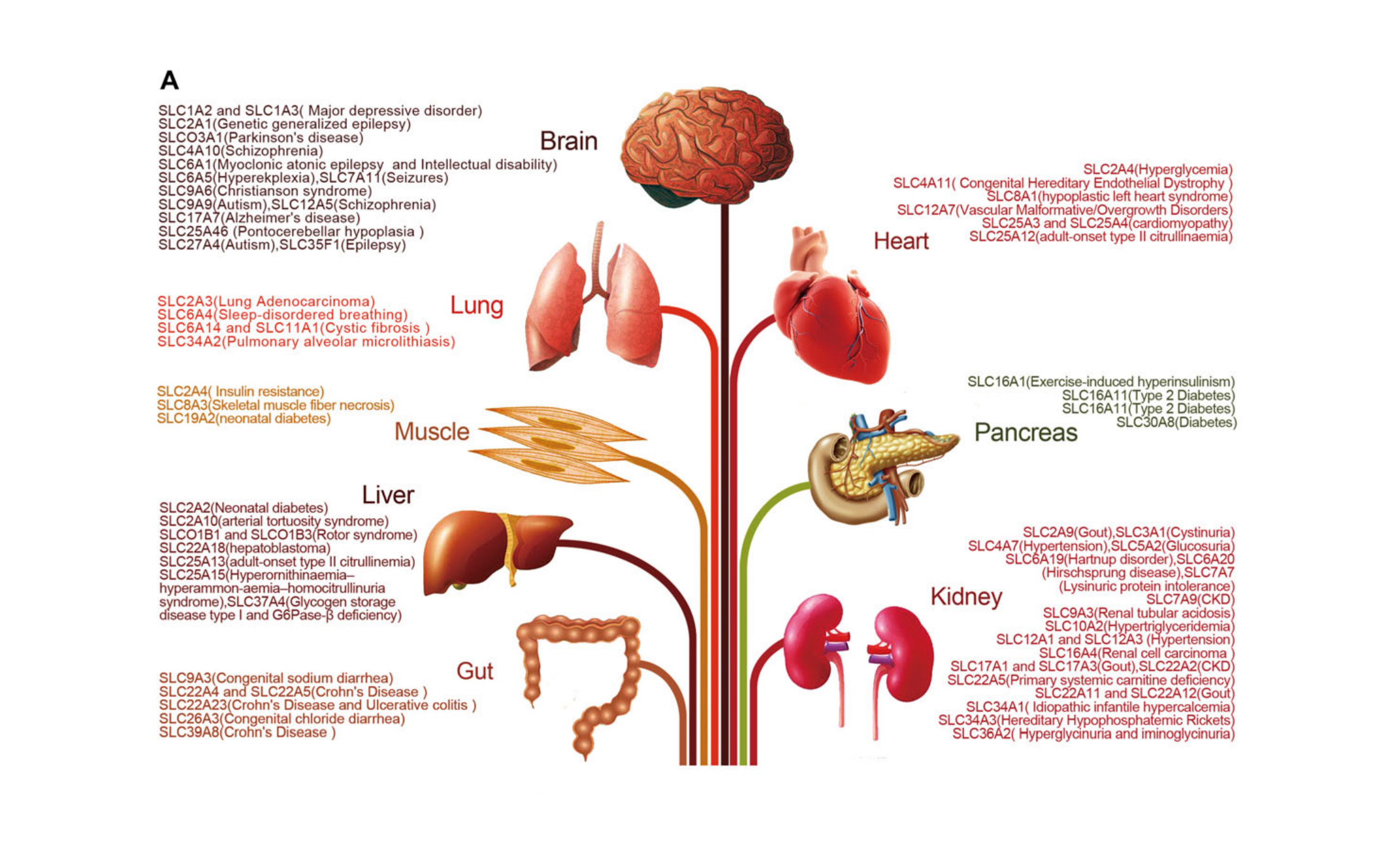
Figure 3A | Association of SLC with disease in different organs (Zhang et al., 2019)
03. A “New Mineral Zone” for Drug Development
Twelve targeted drugs for SLC have been launched, covering areas such as blood sugar control, diuresis, antidepressant therapy, and uric acid reduction:
SGLT2 inhibitors (such as dapagliflozin) → 70 g of glucose excretion per day, a "cross-disciplinary star" in cardio- and renal protection.
SSRI antidepressants (fluoxetine) → Block the reuptake of 5-HT by SLC6A4, increasing synaptic cleft concentration.
Loop diuretics (bumetanide) → Inhibit SLC12A2 (NKCC2), rapidly lowering blood pressure and intracranial pressure.
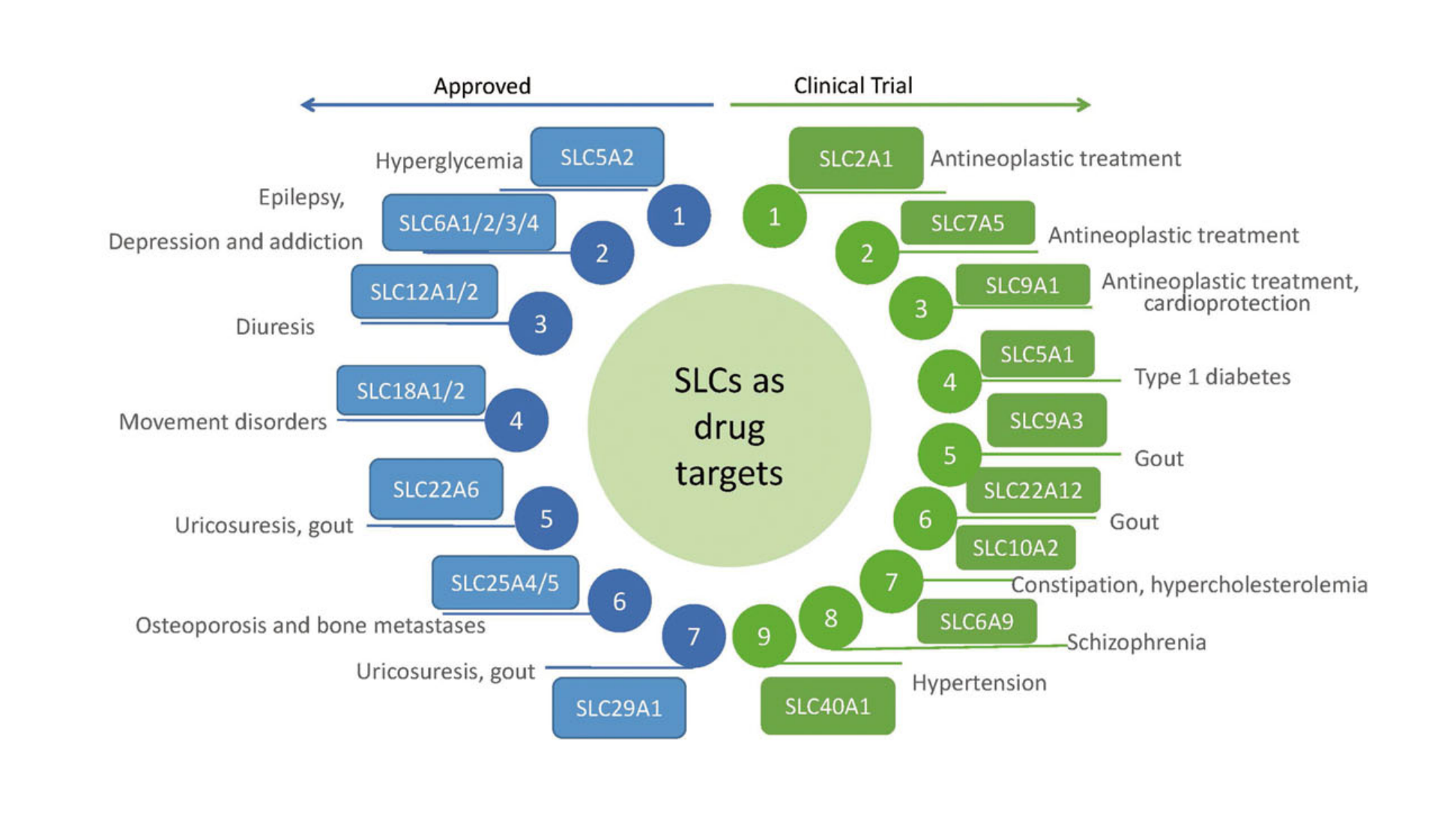
Figure 3B | Marketed and investigational SLC targeted drugs (Zhang et al., 2019)
04 Future Outlook: From "Transportation" to "Precision Nutrition"
With the widespread adoption of CRISPR and organoid technologies, scientists are constructing multi-gene knockout animal models to analyze the "cooperative-compensatory" network among lysosomal SLCs. Combined with single-cell sequencing, personalized "transporter protein maps" can be created, enabling:
- Customizing dietary amino acid ratios based on intestinal SLC genotypes;
- Predicting the efficacy of hypoglycemic/antihypertensive drugs through renal SLC polymorphisms;
- Targeting lysosomal SLCs to regulate mTOR, delaying aging and tumorigenesis.
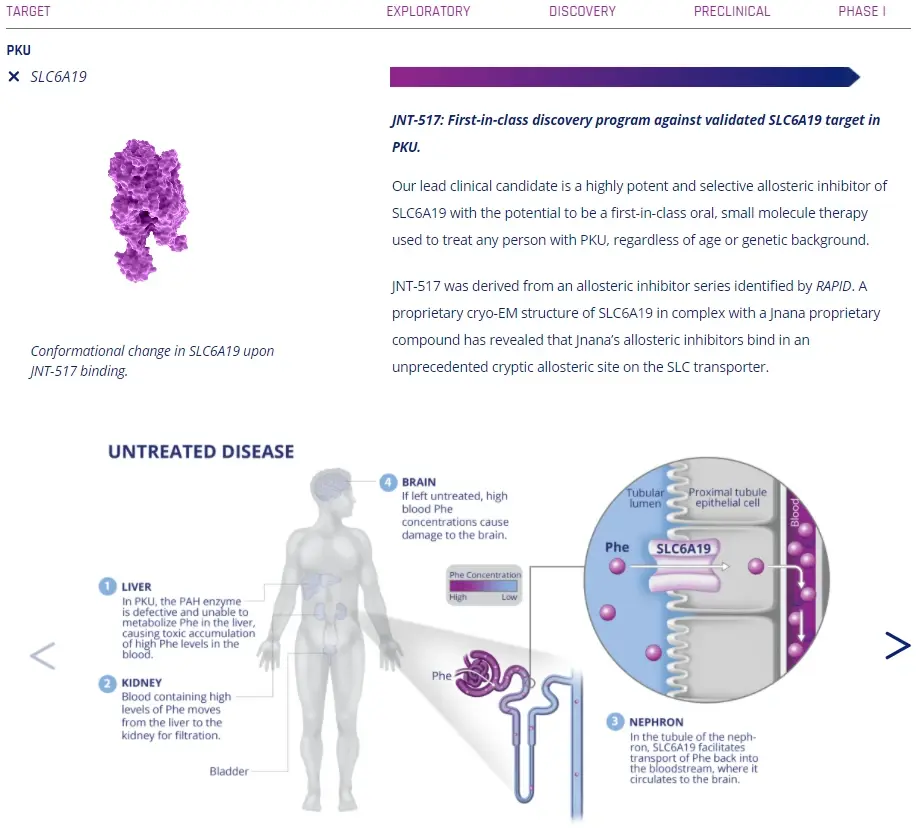
As Boston-based startup Jnana Therapeutics envisions: "Unlocking SLC is unlocking the next goldmine of immune metabolism, lysosomal function, and neuromodulation."
References:
1. Zhang Y. et al. The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. J Mol Cell Biol. 2019.
2. Hediger MA. et al. The ABCs of solute carriers. Pflügers Arch. 2004.
