Guidelines for blood collection in mice and rat
Blood Collection: Another Essential Skill Beyond Drug Administration.
Before performing blood collection, what key points must you know?
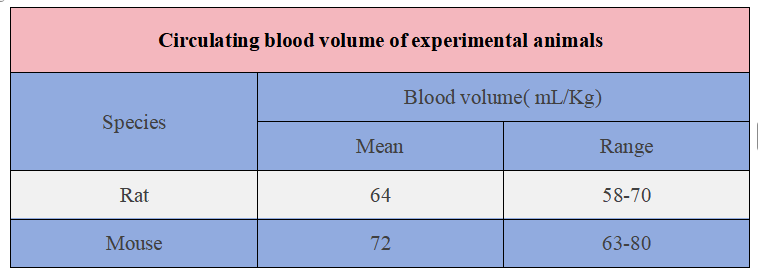
The circulating blood volume of mice is approximately 72 ml/kg. Take an adult mouse of 25 g as an example, its circulating blood volume is 1.8 ml.
The circulating blood volume of rats is approximately 64 ml/kg. Take an adult rat with a mass of 250 g as an example, its circulating blood volume is 16 ml.
Circulating blood volume in rats and mice
After collecting 10% of the circulating blood volume, it takes about two weeks to recover.
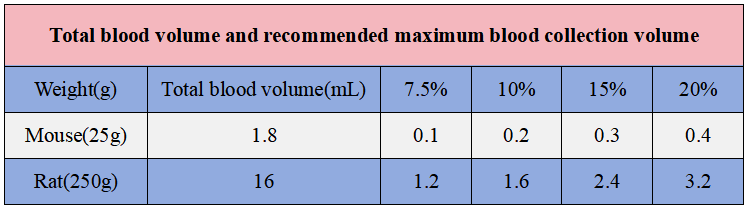
For mice in good condition, the recommended blood collection volume is generally no more than 10% of the circulating blood volume. Studies have shown that experimental animals with blood loss of more than 15% will suffer from hypovolemic shock. Taking an adult mouse weighing 25 g as an example, 10% of the circulating blood volume is approximately 0.2 ml.
Total blood volume and recommended maximum blood collection volume
After blood collection, mice need a period of recovery for their blood indicators to return to normal levels. The following table lists the recovery periods corresponding to different blood collection volumes:
Limit volume and recovery period
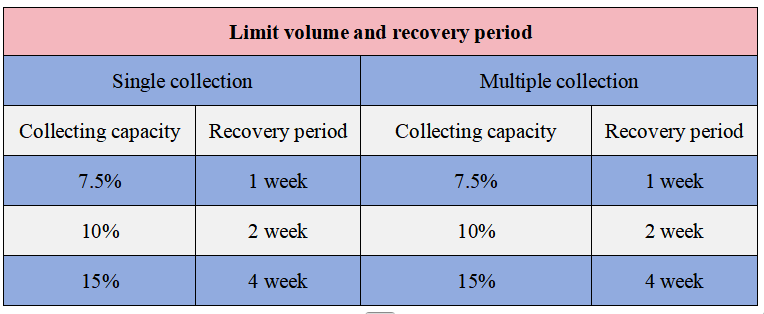
If multiple blood collections are required, the maximum weekly blood collection volume should preferably not exceed 7.5% of the circulating blood volume. The maximum blood collection volume every two weeks shall not exceed 10% of the circulating blood volume.
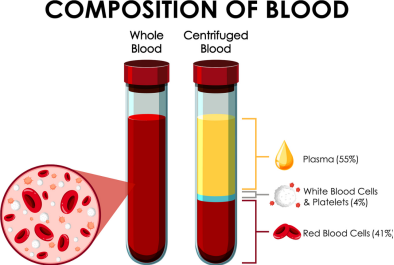
Differences Between Whole Blood, Plasma, and Serum
Whole Blood
Contains all components including blood cells and plasma. Anticoagulants must be added to collection tubes when drawing whole blood. Commonly used anticoagulants include heparin, sodium citrate, and ethylenediaminetetraacetic acid (EDTA). The appropriate anticoagulant should be selected based on research requirements.
Plasma
The cell-free liquid obtained by centrifuging anticoagulated whole blood. Contains fibrinogen, clotting factors and other components.

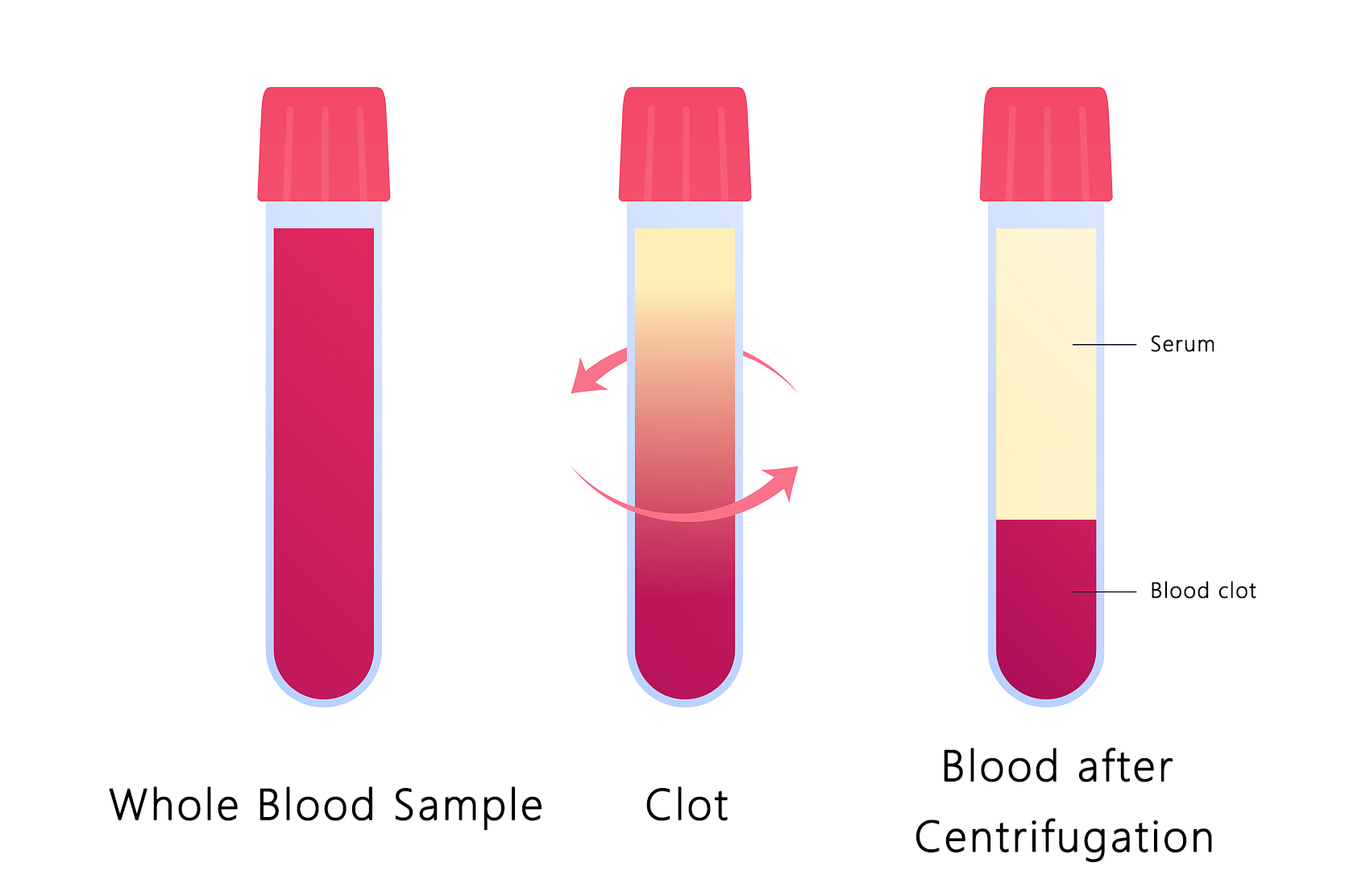
Serum
Serum refers to the pale yellow transparent liquid separated from plasma after fibrinogen has been removed from blood coagulation, or it refers to plasma from which fibrin has been removed.Its main functions are to provide basic nutrients, hormones and various growth factors, binding proteins, contact and extension factors to enable cells to adhere to the surface and avoid mechanical damage, and to play a certain protective role for cells in culture.


The main components of serum
Serum is a very complex mixture formed by removing fibrinogen from plasma. Although the composition of most of its components is known, some remain unclear. Moreover, the composition and content of serum often vary depending on the gender, year and month, physiological condition, and nutritional condition of the blood donor animal.Serum is the gelatinous liquid in plasma without fibrinogen, which plays a role in maintaining the normal viscosity, pH, and osmotic pressure of blood.It is mainly composed of water and various chemical components, which include albumin, α1, α2, β, γ-globulin, triglycerides, total cholesterol, alanine aminotransferase, etc.
Serum contains various plasma proteins, peptides, fats, carbohydrates, growth factors, hormones, inorganic substances, etc. These substances are in physiological balance to promote or inhibit cell growth activity.The general method for separating plasma and serum is to centrifuge at 5000 to 8000 rpm for at least 15 minutes.
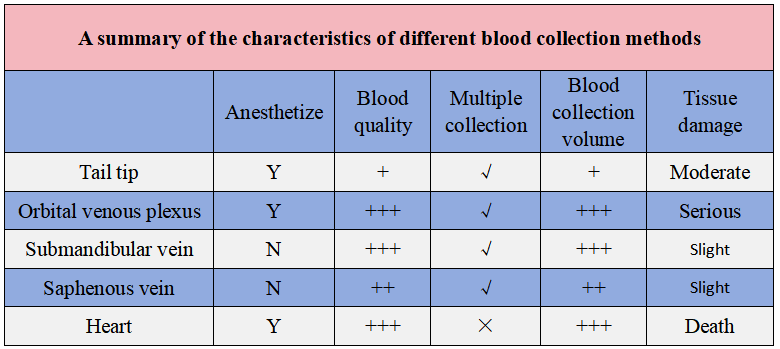
Before collecting blood from the orbital venous plexus, it is necessary to anesthetize the mouse. General anesthesia is usually required. If general anesthesia is not possible, local anesthesia of the eye with proparacaine or tetracaine can be performed before the procedure.The orbital venous plexus blood collection method has the advantages of high blood collection success rate, low mortality rate of mice, fast blood flow, large blood collection volume, and small wound. However, this method cannot collect sterile blood samples, and the blood may be mixed with tissue fluid and glandular secretions in the eye socket.
If multiple blood samplings are required, to ensure the wound healing of the mice, there should be an interval of 10 to 14 days between each sampling. Improper operation or repeated blood samplings from the same eye may cause some complications, such as eye bleeding, inflammation and blindness.
What is a more suitable method for repeated blood collection than blood collection from the orbital venous plexus?
Submandibular vein blood collection or saphenous vein blood collection do not require anesthesia before operation. Moreover, they cause less harm to mice, and the amount of blood collected is comparable to that of the orbital venous plexus blood collection method. The recovery after blood collection is also fast. Therefore, they are more suitable for repeated blood collection than the orbital venous plexus blood collection method.
What other methods are there for blood collection in mice? How should one choose?
Blood collection from different parts of mice can be done in several common ways: tail tip blood collection, orbital venous plexus blood collection, submandibular vein blood collection, saphenous vein blood collection, and cardiac blood collection. How to choose among these methods? We have summarized their respective characteristics.
A summary of the characteristics of different blood collection methods